
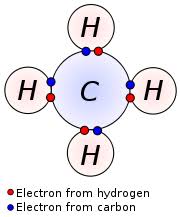
How Many Valence Electrons Does an Element Have? This agrees with the scientific fact that manganese might reach an oxidation state of +7. There are seven valence electrons present outside the core of argon 3d 5 4s 2. In these atoms, the 3d and 4s electrons have the same energy levels and significantly have more energy levels than 3s or 3p electrons. Even though they are not in the outermost shell, d electrons in transition metals act as valence electrons in general.ġs 2 2s 2 2p 6 3s 2 3p 6 3d 5 4s 2 represent the electronic configuration of Manganese (Mn), which can also be written as (Ar) 3d 5 4s 2, where the electronic configuration of (Ar) is similar to the argon which is a noble gas. Therefore, a valence electron belonging to a transition element is at a location other than the noble gases core, in contrast to the main group elements. However, the energy levels of transition elements are (n-1)d, which are approximately close to the ns energy level. This configuration is sometimes expressed in part as Ne for neon (a noble gas), where Ne refers to the core electrons. Another way to show its electron configuration is the short form where its core electrons have the same electronic configuration as this: 3s 2 3p 3. The electronic configuration of phosphorus (P) is 1s 2 2s 2 2p 6 3s 2 3p 3 (long form). In this regard, the quantity of valence electrons a molecule may possess is directly significant and also a function of its electron configuration. The valence electrons of a main-group element are those located in the electronic shell with the largest primary quantum number, n. The highest energy electrons not only determine its valence but also help in determining the chemical behavior of an atom. Valence Electrons and Electronic ConfigurationĪn electronic configuration of an atom refers to the arrangement of electrons in the shells. Nitrogen, on the other hand, may form NH 3, hence it has a valence of 3 and three valence electrons. Historically, the valence of an element was determined by the number of hydrogen atoms it could connect to (which is determined by the number of valence electrons it has available for bonding): for instance, carbon can create CH 4, thus it has a valence of 4 and four valence electrons. Valence refers to an element’s capacity to establish chemical connections with other atoms. (Talk 2022) Figure 1: Diagram of sodium’s electrons. It is located on the outside shell (in this case, the shell resembles a ring). The periodic table can help to understand and determine the valence electrons number an element (particularly a neutral atom of the element) possesses. This also implies that the quantity of valence electrons an element possesses impacts its reactivity, electronegativity, and bonding capacity.įor instance, the valence electron is illustrated in red, which is the simplified picture of sodium’s electrons shown below.


 0 kommentar(er)
0 kommentar(er)
